SSEF Merit Award
Singapore Science and Engineering Fair (SSEF) 2023
Jonathan Koh Zhi Hong and Gu Xinyi, Hwa Chong Institution
Supervisor: A/Prof Nikolai Yakovlev
CH002, Competing mechanisms in the assembly of tannic acid-gelatin multilayers
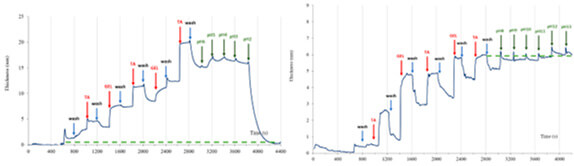
Layer-by-layer (LbL) techniques have been widely applied in the assembly of hydrogen bonded and polyelectrolyte multilayers. These multilayers have shown promising potential in the synthesis of selectively permeable biofilms and capsules that can be utilised for drug delivery. In this study, tannic acid (TA), gelatin type A or B (GELA, GELB) were assembled alternatingly via LbL technique. Changes in thickness and TA-GELA and TA-GELB multilayers during assembly and acid or base treatment were monitored in situ using precision ellipsometry (PREL). TA-GELA and TA-GELB multilayers were assembled with GEL of pH 3, 5, 7, 9. The thickness of the multilayers exhibited surprisingly similar trends with GEL of pH 7 displaying the greatest thickness followed by pH 5. To explain that, a model has been proposed of the varying strength of electrostatic repulsion between TA and GEL in different pH conditions. The competition between the repulsive forces and the hydrogen bonds over the attachment of the layers arises. The outcome of this competition hence determines the stability of TA-GEL multilayers in acidic and basic conditions. TA-GELA and TA-GELB multilayers upon acid or base treatment exhibited completely opposite behaviours. Under acidic conditions, TA-GELA multilayers where unstable at pH 2 while TA-GELB multilayers were stable. Under basic conditions, TA-GELB multilayers were unstable at pH 12 while TA-GELA multilayers were stable. Hence by altering pH at LbL, the stability of TA-GEL multilayers in acidic or alkaline conditions can be controlled, illustrating promising potential in controlled drug release applications.