Novel immunoassays for SARS-CoV-2 virus and anti-SARS-CoV-2 antibody level developed
A research team led by Professor Yan Jie from the Mechanobiology Institute and Department of Physics, National University of Singapore, has developed a novel immunoassay for detecting the presence of a specific biomolecular target with high sensitivity and specificity.

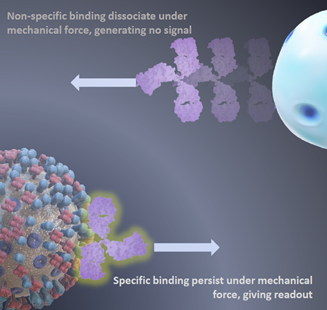
Unlike most of the current immunoassays that rely on detecting the binding affinity difference between specific and nonspecific molecular interactions, the assay developed by the team is based on the difference in the force-dependent dissociation kinetics between specific and nonspecific biomolecular complexes. The force quickly removes the non-specifically formed complexes, leaving the remaining ones which are mainly the complexes formed with specific biomolecular interactions. This mechanism leads to mechanically enhanced specificity, with the signal-to-noise ratio increasing exponentially with time. The team also developed methods to detect the remaining biomolecular complexes at a near-single-molecule level, achieving high detection sensitivity. The technologies behind the immunoassay have led to the filing of two Singapore Provisional Patents, both currently under the Patent Cooperation Treaty conversion in seeking patent protection internationally.
The immunoassay and the underlying technologies have a wide range of applications. Based on this technology, the team has developed rapid test kits for SARS-CoV-2 infection and antibodies produced from a past SARS-CoV-2 infection or from recent vaccination against SARS-CoV-2. Each test can be completed within 30 minutes, requiring only very small sample volume (< 30 uL). By mixing the SARS-CoV-2 nucleocapsid proteins with saliva or mid-turbinate swab sample, laboratory results have shown that their SARS-CoV-2 test kit can detect the nucleocapsid proteins at a concentration 100 times lower than most of the currently applied SARS-CoV-2 rapid test kits. Their antibody test kit can detect the presence of IgG antibody against the receptor binding domain (RBD) of SARS-CoV-2 within seven days after receiving the first dose of Pfizer or Moderna vaccine, and quantify the dynamics of the level of RBD-targeting antibodies post vaccination. The team has been working with industrial collaborators for the commercialization of these test kits.